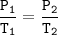Chemistry, 02.02.2021 20:20, anastasiasam1916
A certain amount of gas at 25°C and at a pressure of 0.800 atm is contained in a glass vessel. Suppose that the vessel can withstand a pressure of 2.00 atm. What is the maximum temperature in Kelvin that the gas can have without bursting the vessel?
a. 745 K
b. 62.5 K
c. 298 K
d. 760 K

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 22:40, hannahkharel2
Maleic acid is a carbon-hydrogen-oxygen compound used in dyeing and finishing fabrics and as a preservative of oils and fats.* in a combustion analysis, a 1.054-g sample of maleic acid yields 1.599 g of co_2 and 0.327 g of h2o.* when 0.609 g of maleic acid is dissolved in 25.00 g of glacial acetic acid, the freezing point drops by 0.82 c. maleic acid does not ionize in glacial acetic acid, which has a freezing-point depression constant of k_{\rm f}=3.90~^\circ {\rm c}/m.* in a titration experiment, a 0.4250- g sample of maleic acid is dissolved in water and requires 34.03 ml of 0.2152 m koh for its complete neutralization.* the ph of 0.215 g of maleic acid in 50.00 ml of aqueous solution is found to be 1.80.part adetermine the empirical formula for maleic acid. enter the atoms in order ofc, h, and o. part bdetermine the molecular formula for maleic acid. enter the atoms in order of c, h, and o. express your answer as a chemical formula. part cbased on the titration data, how many ionizable h atoms are in each molecule of maleic acid, c4h4o4? express your answer as an integer. i got the part a and b which is cho and c4h4o4 respectively, can anyone me for part c? the below is what i have so far from other qa'sfinding the empirical formula for maleic acid, needs this info"a combustion analysis a 1.054-g sample of maleic acid yields 1.599g of and 0.327g of ".the rest of your data is used to find molar mass, molecular formula, equivalent mass, # of h+ ions / molecule & katypically this should be: a 1.054-g sample of maleic acid yields 1.599g of co2 and 0.327g of h2osofind mass using molar masses1.599g of co2 @ (12.01 g carbon) / (44.01 g co2) = 0.436 grams of carbon0.327g of h2o @ (2.016 g hydrogen) / (18.01 g/mol h2o) = 0.037 grams of hydrogen1.054-g sample - 0.436 grams of carbon - 0.037 grams of hydrogen = 0.581 grams of oxygennow use molar masses to find moles0.436 grams of carbon @ 12.01 g/mol = 0.0363 mol c0.037 grams of hydrogen @ 1.008 g/mol = 0.0367 mol h0.581 grams of oxygen @ 16.00 g/mol = 0.0363 mol oyour empirical formula ischo and molecular is c4h4o4
Answers: 3

Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 07:30, libbymcvay
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
Do you know the correct answer?
A certain amount of gas at 25°C and at a pressure of 0.800 atm is contained in a glass vessel. Suppo...
Questions in other subjects:

Mathematics, 08.01.2021 19:40

English, 08.01.2021 19:40

Spanish, 08.01.2021 19:40


English, 08.01.2021 19:40


Mathematics, 08.01.2021 19:40

Social Studies, 08.01.2021 19:40


Mathematics, 08.01.2021 19:40