
Chemistry, 02.02.2021 17:40, oKINGDEROo
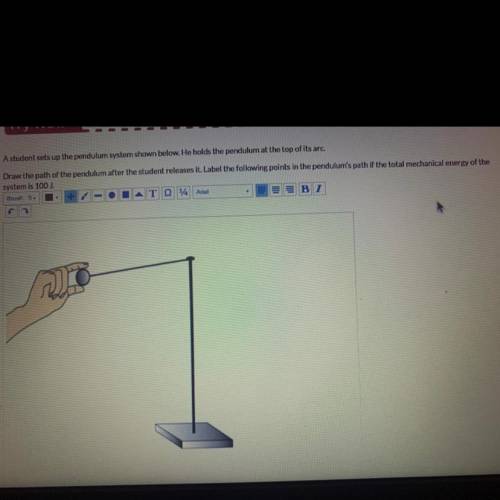
A student sets up the pendulum system shown below. He holds the pendulum at the top of its arc.
Draw the path of the pendulum after the student releases it. Label the following points in the pendulum's path if the total mechanical energy of the
system is 100 J.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1


Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
Do you know the correct answer?
A student sets up the pendulum system shown below. He holds the pendulum at the top of its arc.
Dra...
Questions in other subjects:

English, 17.11.2020 20:20

Chemistry, 17.11.2020 20:20

Biology, 17.11.2020 20:20

Mathematics, 17.11.2020 20:20

Mathematics, 17.11.2020 20:20










