
Chemistry, 02.02.2021 16:50, daeshawnc14
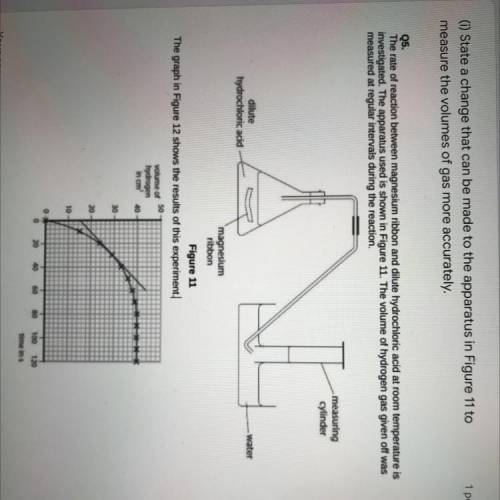
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more accurately.
Q5.
The rate of reaction between magnesium ribbon and dilute hydrochloric acid at room temperature is
investigated. The apparatus used is shown in Figure 11. The volume of hydrogen gas given off was
measured at regular intervals during the reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2


Do you know the correct answer?
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more...
Questions in other subjects:

Mathematics, 27.08.2019 04:50


Physics, 27.08.2019 04:50



Physics, 27.08.2019 04:50




Mathematics, 27.08.2019 04:50






