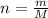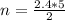Chemistry, 02.02.2021 04:00, ExperimentsDIYS
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502 --> 4CO2 + 2H20
When 2.40mol C2H2 reacts with 7.40mol O2,
a. Which reactant is the limiting reactant?
b. How many grams of water can be produced by the reaction?
c. How many moles of the excess reactant remains?
I NEED THIS ANSWER QUICKLY IF YOU CAN ONLY DO B THATS FINE THANK YOU

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
Do you know the correct answer?
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502...
Questions in other subjects:


English, 28.05.2021 19:30


Mathematics, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30


Mathematics, 28.05.2021 19:30


Biology, 28.05.2021 19:30

Mathematics, 28.05.2021 19:30


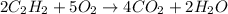
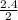 = 1.2
= 1.2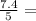 1.48
1.48