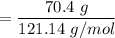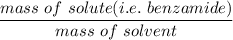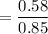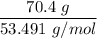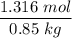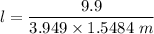Chemistry, 01.02.2021 20:40, alyonaprotopopova
When 70.4 g of benzamide (C7H7NO) are dissolved in 850. g of a certain mystery liquid X, the freezing point of the solution is 2.7 C lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (NH CI) are dissolved in the same mass of X, the freezing point of the solution is 9.9 °C lower than the freezing point of pure X.
Required:
Calculate the van't Hoff factor for ammonium chloride in X.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 06:40, Science2019
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 10:30, oglejack6138
How is it possible for someone to put an ear to a wall and hear someone in the next room? a. sound waves can travel though solids. b. the waves travel from room to room via air. c. there must be some air in the wall so the sound can travel through it. d. sound waves change to electromagnetic waves and then back again.
Answers: 1
Do you know the correct answer?
When 70.4 g of benzamide (C7H7NO) are dissolved in 850. g of a certain mystery liquid X, the freezin...
Questions in other subjects:


Chemistry, 07.03.2020 02:30







Mathematics, 07.03.2020 02:30