CH4 + 202 → CO2 + 2H2O
How many moles of O2 needed to react with 32 grams of CH4?...

Chemistry, 30.01.2021 19:00, winstonbendariovvygn
CH4 + 202 → CO2 + 2H2O
How many moles of O2 needed to react with 32 grams of CH4?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1


Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 21.09.2019 23:30


History, 21.09.2019 23:30



Mathematics, 21.09.2019 23:30


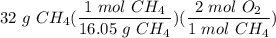 Divide/Multiply:
Divide/Multiply: 




