
Chemistry, 30.01.2021 16:10, kaylee0424
PLEASE HELP ME
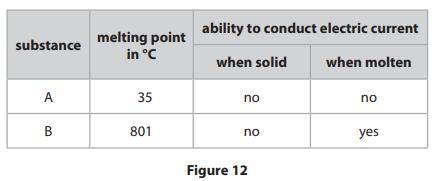
Figure 12 shows the melting points of two substances, A and B, and the abilities
of the substances to conduct an electric current when solid and when molten.
One of the substances has an ionic structure and one has a simple molecular,
covalent structure.
Explain, in terms of bonding and the forces between the particles, the relative
melting points and abilities to conduct the electric current of substances A and B.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2


Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
Do you know the correct answer?
PLEASE HELP ME
Figure 12 shows the melting points of two substances, A and B, and the abilities
Questions in other subjects:

Physics, 25.07.2020 20:01






History, 25.07.2020 20:01


Mathematics, 25.07.2020 20:01






