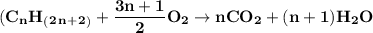Chemistry, 30.01.2021 09:00, lhecker007
What would be the balanced chemical equation for the complete combustion of butane ( C4H10 .)
Select one:
a. 2 C4H10 + 5 CO2 --> 8 NiO2 + 5 H2O
b. 2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
c. 2 C4H10 + 13 CO2 --> 8 O2 + 10 H2O
d. 4 C4H10 + 6 H2O --> 8 CO2 + 5 O2

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:30, savag3jitt
In all living cells dna controls cellular activities by
Answers: 1


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
Do you know the correct answer?
What would be the balanced chemical equation for the complete combustion of butane ( C4H10 .)
Sel...
Questions in other subjects:


Computers and Technology, 09.12.2020 18:30





English, 09.12.2020 18:30



Mathematics, 09.12.2020 18:30