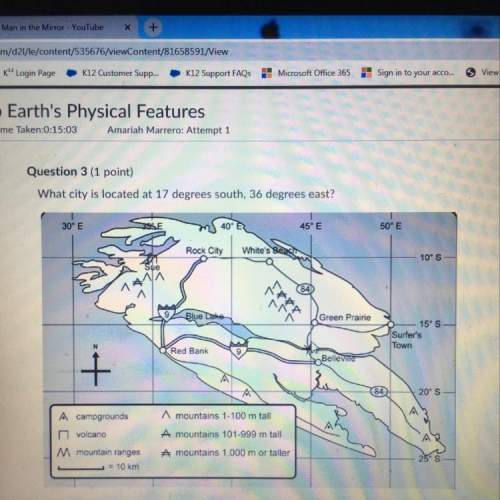
Chemistry, 30.01.2021 09:00, ashiteru123
Determine if the individual bonds in each compound are polar or nonpolar.
Include electronegativity values and the difference to show your work.
EXAMPLE: Se=2.4 O= 3.5 3.5-2.4=1.1 Polar
1. SCl2
2. H2S
3. CF4
4. PC15
5. CHA
6. CaBr2
7. SF2
8. CO2
9. NH3
10. Na s

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3
Do you know the correct answer?
Determine if the individual bonds in each compound are polar or nonpolar.
Include electronegativity...
Questions in other subjects:


Mathematics, 29.01.2021 18:20




Biology, 29.01.2021 18:20


Arts, 29.01.2021 18:20