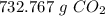Chemistry, 30.01.2021 07:50, stupidjew5496
2C3H7OH + 9O2 --> 6CO2 + 8H2O Determine the number of grams of CO2 produced from the reaction of 5.55 moles of C3H7OH

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Do you know the correct answer?
2C3H7OH + 9O2 --> 6CO2 + 8H2O
Determine the number of grams of CO2 produced from the reaction of...
Questions in other subjects:

English, 27.02.2021 14:20



Social Studies, 27.02.2021 14:20


Biology, 27.02.2021 14:20

Social Studies, 27.02.2021 14:20


History, 27.02.2021 14:20


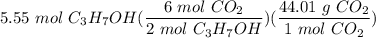 Multiply/Divide:
Multiply/Divide: