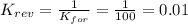I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure would
N2(g)+3H2(g)⇄2NH3(g)
a. increase [H2]
b. increase [NH3]
c. increase [N2]
d. have no effect
2. If K=100, then the value of K for the reverse reaction is
a. the same value
b. can only be determined by experimentation
c. the negative of the value for the forward reaction
d. 0.01

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Do you know the correct answer?
I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure woul...
Questions in other subjects:





History, 25.11.2021 07:50

Mathematics, 25.11.2021 07:50

English, 25.11.2021 07:50