
Chemistry, 30.01.2021 05:40, dominguezjose625
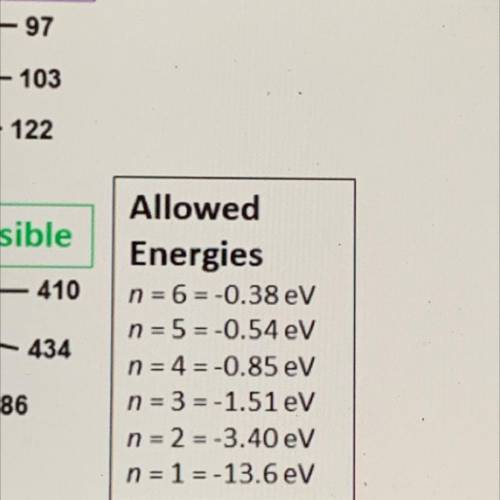
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers
are the wavelengths in nanometers of the associated electromagnetic radiation.
Which wavelength is associated with a photon energy of 1.13 electron volts?
Bohr Model of the Hydrogen Atom
ultraviolet
infrared
-97
103
1,094
122
1,282
1,875
visible
410
434
Allowed
Energies
n = 6-0.38 eV
n = 5=-0.54 eV
n = 4 = -0.85 eV
n = 3 = -1.51 eV
n = 2 = -3.40 eV
n = 1 = -13.6 eV
486
656
In orbits
n orbits

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, EllaLovesAnime
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 08:00, 91miketaylor
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3
Do you know the correct answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions in other subjects:





Mathematics, 18.11.2020 23:20










