
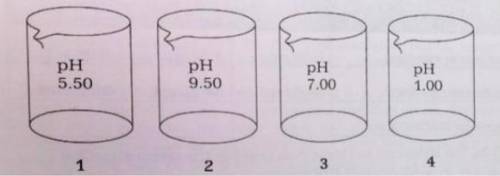
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) The Kb for ammonia is 1.8 x 10^-5
(i) Which beaker is most likely to contain NH3(aq)? Provide a chemical equation to explain your answer.
(ii) Calculate the molarity of the solution in the beaker that you chose for (i).
(b) If the contents of beakers 3 and 4 are poured together and mixed thoroughly, what will be the resulting pH?
(c) Explain how it is possible that beakers 1 and 4 are acids with equal molarities.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2
Do you know the correct answer?
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) T...
Questions in other subjects:


World Languages, 02.08.2019 15:30

Biology, 02.08.2019 15:30

Mathematics, 02.08.2019 15:30


Chemistry, 02.08.2019 15:30


Physics, 02.08.2019 15:30

Mathematics, 02.08.2019 15:30







