
Natural gas is stored in a spherical tank at a temperature of 13°C. At a given initial time, the pressure in the tank is 117 kPa gage, and the atmospheric pressure is 100 kPa absolute. Some time later, after considerably more gas is pumped into the tank, the pressure in the tank is 212 kPa gage, and the temperature is still 13°C. What will be the ratio of the mass of natural gas in the tank when p = 212 kPa gage to that when the pressure was 117 kPa gage?
For this situation in which the tank volume is the same before and after filling, which of the following is the correct relation for the ratio of the mass after filling M2 to that before filling M1 in terms of gas temperatures T1 and T2 and pressures p1 and p2?
a. M2/M1= p2T2/p1T1
b. M2/M1= p1T2/p2T1
c. M2/M1= p2T1/p1T2
d. M2/M1= p1T1/p2T2
1. What is the absolute pressure in the tank before filling?
2. What is the absolute pressure in the tank after filling?
3. What is the ratio of the mass after filling M2 to that before filling M1 for this situation?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2


Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
Do you know the correct answer?
Natural gas is stored in a spherical tank at a temperature of 13°C. At a given initial time, the pre...
Questions in other subjects:

English, 20.07.2021 16:40


Mathematics, 20.07.2021 16:50


Mathematics, 20.07.2021 16:50


Chemistry, 20.07.2021 16:50

Mathematics, 20.07.2021 16:50



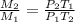 )
)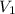 =
= 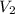
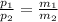
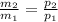
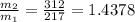 ≈ 1.44
≈ 1.44 ∝ M
∝ M




