Chemistry, 29.01.2021 16:50, mcaninch36
Consider the combustion of propane: A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from L to L by the addition of J energy as heat. Assume that all the heat comes from the combustion of propane. What mass of propane must be burned to furnish this amount of energy assuming the heat transfer process is 50.% efficient?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
Do you know the correct answer?
Consider the combustion of propane: A balloon is being inflated to its full extent by heating the ai...
Questions in other subjects:


History, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30


Mathematics, 09.07.2019 06:30





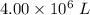 to
to 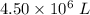 .
.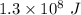
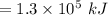
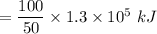
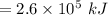
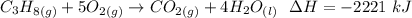
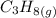
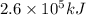 will produce
will produce