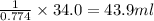Chemistry, 29.01.2021 16:30, elisakgarcia2007
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculate the volume of a saturated solution of X in water that would contain 34.0g of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:40, marknjenbennetp3j1v1
Listen base your answer to the question on the information below. propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below. c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol. what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
Do you know the correct answer?
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculat...
Questions in other subjects:


Mathematics, 17.07.2019 21:50

History, 17.07.2019 21:50

History, 17.07.2019 21:50

Chemistry, 17.07.2019 21:50

Mathematics, 17.07.2019 21:50

Social Studies, 17.07.2019 21:50

History, 17.07.2019 21:50

Biology, 17.07.2019 21:50


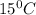 = 0.776 g/ml
= 0.776 g/ml