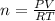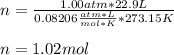Chemistry, 29.01.2021 14:00, aisatubrodie4626
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
If 22.9L of an ideal gas was collected at STP. How many moles of the gas were present?
A. 1.02 moles
B. 5.99 moles
C. 3.05 moles
D. 2.74 moles


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
Do you know the correct answer?
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
Questions in other subjects:

Mathematics, 26.10.2020 19:40

History, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40

Social Studies, 26.10.2020 19:40


Computers and Technology, 26.10.2020 19:40

Biology, 26.10.2020 19:40

English, 26.10.2020 19:40

Chemistry, 26.10.2020 19:40