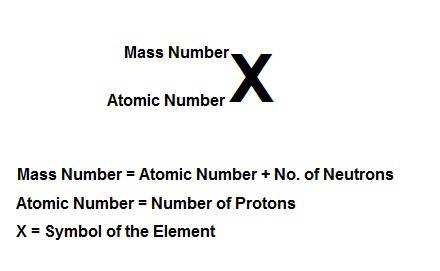
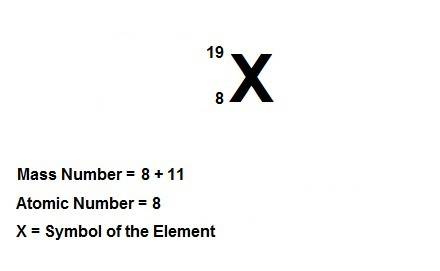Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutrons. click on the “templates” button template button and make use of the "stacked super/subscript" button button for entering stackes super/subscripts for entering the mass number and atomic number of the isotope. express your answer as an isotope.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Do you know the correct answer?
Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutro...
Questions in other subjects:

Mathematics, 29.01.2020 07:09


Mathematics, 29.01.2020 07:09


Social Studies, 29.01.2020 07:09


Chemistry, 29.01.2020 07:09

History, 29.01.2020 07:09


History, 29.01.2020 07:09