
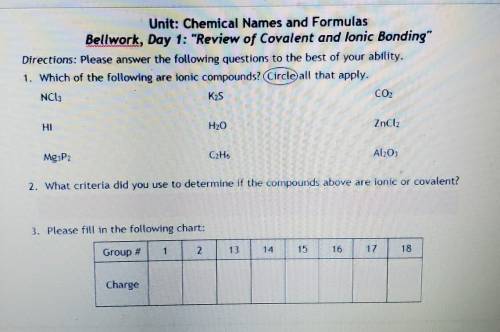
Unit: Chemical Names and Formulas Bellwork, Day 1: "Review of Covalent and lonic Bonding" Directions: Please answer the following questions to the best of your ability. 1. Which of the following are ionic compounds? (Circle all that apply. NCIE Kas CO2 H20 ZnCl2 Mg3P2 C. H. Al2O3 2. What criteria did you use to determine if the compounds above are ionic or covalent? 3. Please fill in the following chart: Group F 1 15 Charge

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
Do you know the correct answer?
Unit: Chemical Names and Formulas Bellwork, Day 1: "Review of Covalent and lonic Bonding" Directions...
Questions in other subjects:



History, 02.09.2019 02:10



Biology, 02.09.2019 02:10


Chemistry, 02.09.2019 02:10


Mathematics, 02.09.2019 02:10






