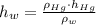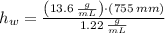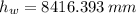Chemistry, 28.01.2021 22:20, Hazeleyes13
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would the liquid level be in this barometer if the atmospheric pressure was 755 torr? (Mercury has a density of 13.6 g/mL.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3



Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would...
Questions in other subjects:


Mathematics, 19.04.2021 16:50

Mathematics, 19.04.2021 16:50



Computers and Technology, 19.04.2021 16:50

History, 19.04.2021 16:50


Spanish, 19.04.2021 16:50


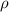 ), measured in grams per mililiter, and height of fluid (
), measured in grams per mililiter, and height of fluid (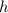 ), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship:
), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship: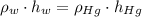 (1)
(1)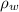 ,
, 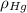 - Densities of fluid and mercury, measured in grams per mililiter.
- Densities of fluid and mercury, measured in grams per mililiter.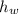 ,
, 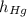 - Heights of fluid and mercury columns, measured in milimeters.
- Heights of fluid and mercury columns, measured in milimeters.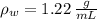 ,
, 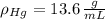 and
and 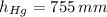 , then the liquid level of this barometer is:
, then the liquid level of this barometer is: