
Chemistry, 28.01.2021 21:20, pandagirl710
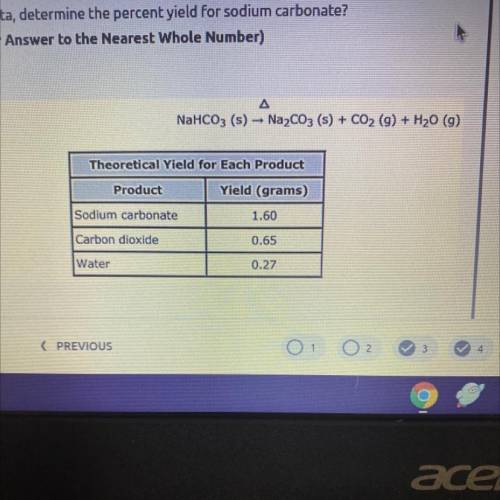
BRAINLIEST PLEASEEE HELPLP 5. In a lab experiment, 2.5 grams of sodium bicarbonate is heated and decomposed into
sodium carbonate, carbon dioxide, and water vapor when heated. The actual yield of
sodium carbonate produced in the experiment is 2.04 grams. The theoretical yield of
each product is recorded in the data table below.
Using this data, determine the percent yield for sodium carbonate?
(Round Your Answer to the Nearest Whole Number)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, nnaomii
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2


Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
Do you know the correct answer?
BRAINLIEST PLEASEEE HELPLP 5. In a lab experiment, 2.5 grams of sodium bicarbonate is heated and dec...
Questions in other subjects:




English, 05.04.2021 20:20

Health, 05.04.2021 20:20

Health, 05.04.2021 20:20

History, 05.04.2021 20:20

English, 05.04.2021 20:20







