Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
Do you know the correct answer?
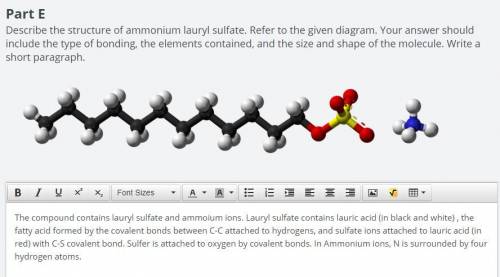
Explain how the structure of ammonium lauryl sulfate, as described in parts E and F, produces the pr...
Questions in other subjects:

Mathematics, 18.03.2021 01:20

English, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20