
Chemistry, 27.01.2021 20:40, GachaSkylarUwU
A main group element with the valence electron configuration 3s23p1 is in periodic group . It forms a monatomic ion with a charge of . A main group element with the valence electron configuration 4s24p5 is in periodic group . It forms a monatomic ion with a charge of .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
Do you know the correct answer?
A main group element with the valence electron configuration 3s23p1 is in periodic group . It forms...
Questions in other subjects:






Mathematics, 04.04.2021 01:00




History, 04.04.2021 01:00


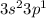
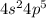

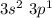 main group component to valence electronic structure (IIIA group) and Aluminum (Al). The monatomic ion forms a load of (+3)
main group component to valence electronic structure (IIIA group) and Aluminum (Al). The monatomic ion forms a load of (+3)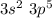 (Cl). It constitutes a monatomic ion with a load of (-1).
(Cl). It constitutes a monatomic ion with a load of (-1).



