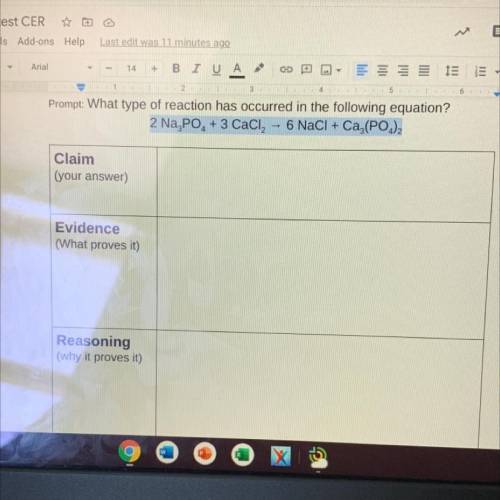
What type of reaction occur in 2Na3PO4 + 3CaCl2 --> 6NaCl + Ca3(PO) 2?
...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
Do you know the correct answer?
Questions in other subjects:



Business, 13.02.2020 22:00