
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performing
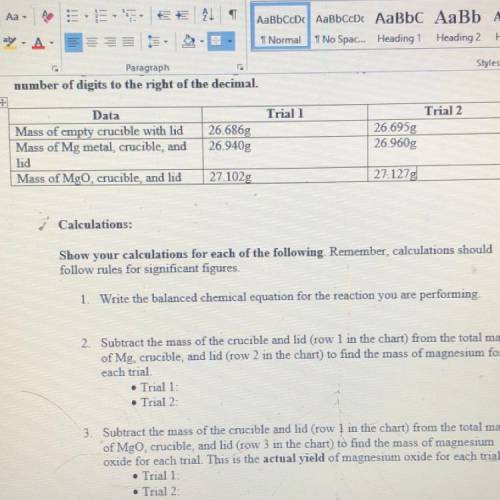
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and kid (row 2 in the chart) to find the mass of magnesium for each trial
Trial 1:
Trial 2:
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual YIELD of magnesium oxide for each trial
Trial 1:
Trial 2:
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial
Trial 1:
Trial 2:
6. Determine the average percent yield of MgO for 2 Trials
CONCLUSION
Write a conclusion statement that addresses the following questions
• Explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
• What sources of error may have contributed to the percent yield not being 100%?
(Think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.)
• How do you think the investigation can be explored further?
POST LAB REFLECTION QUESTIONS
1. When conducting this experiment, some procedures call for heating the substance several times and recording the mass after each heating, continuing until the mass values are constant. Explain the purpose of this process and how it might reduce errors.
2. Your company currently uses a process with a similar cost of materials that has an average percent yield of 91%. If the average percent yield of this process is higher than that, this could save the company money. What is your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
Do you know the correct answer?
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performi...
Questions in other subjects:


Mathematics, 27.06.2020 22:01













