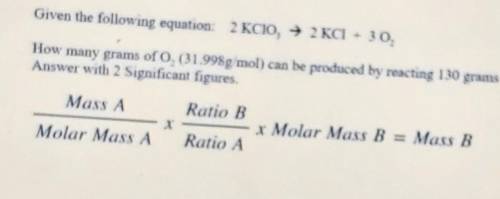Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
Do you know the correct answer?
Given the following equation: 2KCIO, — 2 KCl + 30. How many grams of O, (ch31.998g mol) can be produ...
Questions in other subjects:


History, 10.11.2020 21:00


Health, 10.11.2020 21:00


English, 10.11.2020 21:00



Social Studies, 10.11.2020 21:00

History, 10.11.2020 21:00