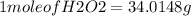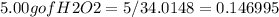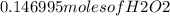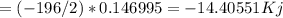Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l)...

Chemistry, 19.01.2020 09:31, calvinclifton
Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l) 2h2o(l) + o2(g) enthalpy=-196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 15:00, alondrabdiaz586
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3

Chemistry, 23.06.2019 16:30, ejcastilllo
There is a set up transformer that doubles the voltage. if the primary coil has a voltage of 10 v
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Spanish, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20




Physics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20


Chemistry, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20