
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
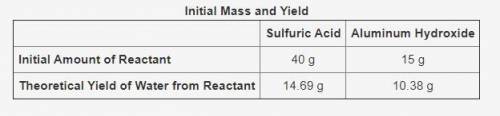
The table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory.
What is the approximate amount of the leftover reactant?
11.73 g of sulfuric acid
10.33 g of sulfuric acid
11.12 g of aluminum hydroxide
13.67 g of aluminum hydroxide

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)...
Questions in other subjects:

Mathematics, 09.10.2019 11:50

Mathematics, 09.10.2019 11:50

Biology, 09.10.2019 11:50


History, 09.10.2019 11:50


History, 09.10.2019 11:50

Mathematics, 09.10.2019 11:50

Mathematics, 09.10.2019 11:50

Business, 09.10.2019 11:50






