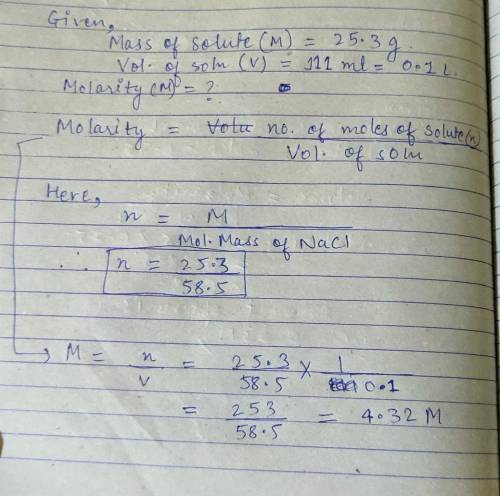Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
Do you know the correct answer?
What is the molarity of a solution that contains 25.3 g of NaCl in 111 mL of solution?
-14.6 M
Questions in other subjects:


Physics, 30.08.2020 02:01

Social Studies, 30.08.2020 02:01




Mathematics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01