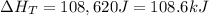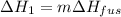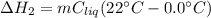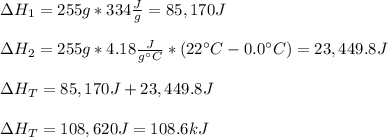Chemistry, 26.01.2021 06:20, pleasehelp5334me2
A 255 g sample of ice at 0.0 0C was melted and its temperature increased to 22 0C. What was the amount of heat (q) transferred?
Heat of fusion for water (ΔHfus) is 334 j/g
The specific heat of water is 4.18 J/g • 0C
This is a two step process. What steps are necessary to solve the problem?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
Do you know the correct answer?
A 255 g sample of ice at 0.0 0C was melted and its temperature increased to 22 0C. What was the amou...
Questions in other subjects:






History, 12.07.2019 09:00