
Chemistry, 26.01.2021 05:40, kenneth0125
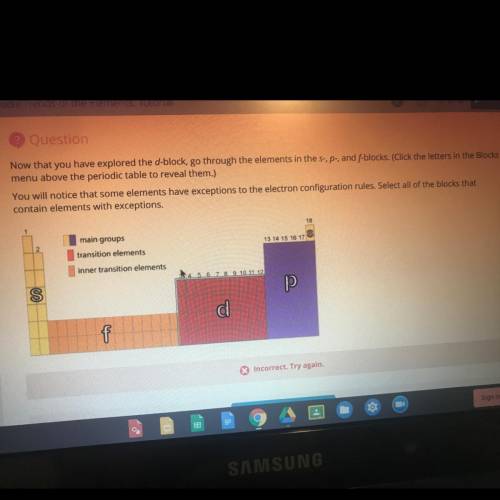
Now that you have explored the d-block, go through the elements in the s-, p-, and f-blocks. You will notice that some elements have exceptions to the electron configuration rules. Select all of the blocks that contain elements with exceptions.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, whrjegt4jrnfdvj
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
Do you know the correct answer?
Now that you have explored the d-block, go through the elements in the s-, p-, and f-blocks.
You wi...
Questions in other subjects:

English, 03.07.2019 04:00


Arts, 03.07.2019 04:00



Mathematics, 03.07.2019 04:00









