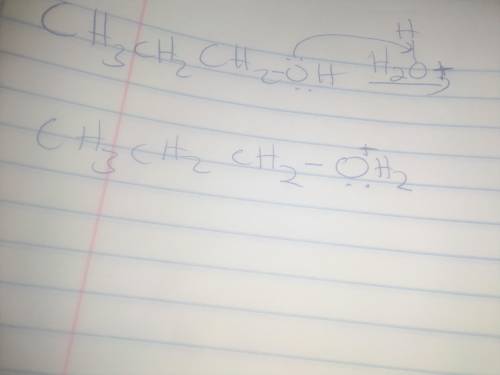When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium ion. There is a scheme of a reversible chemical reaction. The substrates are CH3CH2CH2OH molecule and H with a charge of 1 plus ion. The product is CH3CH2CH2OH2 with a charge of 1 plus ion. Oxygen atom in CH3CH2CH2OH molecule has 2 lone pairs. Oxygen atom in CH3CH2CH2OH2 with a charge of 1 plus ion has a lone pair. All bonds are single. Using the curved arrow formalism, show how this process most likely occurs.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
Do you know the correct answer?
When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium i...
Questions in other subjects:

Mathematics, 03.12.2019 06:31

Computers and Technology, 03.12.2019 06:31

Chemistry, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31


Chemistry, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31



Biology, 03.12.2019 06:31