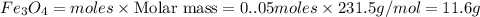Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8....

Chemistry, 26.01.2021 01:10, lazavionadams81
Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8.4 g of Fe, how many of Fe 3O4 are formed?
a) Calculate the limiting reactant
b) Calculate the number of grams of Al produced.
c) Calculate the number of grams of Fe3O4 produced.
d) Calculate the percent yield if 10g of Fe O4 were obtained?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
Do you know the correct answer?
Questions in other subjects:



Social Studies, 18.02.2021 18:50

Mathematics, 18.02.2021 18:50


History, 18.02.2021 18:50


Mathematics, 18.02.2021 18:50


Biology, 18.02.2021 18:50


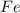 is the limiting reagent
is the limiting reagent 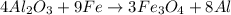
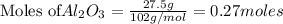
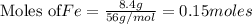
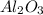
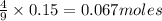 of
of 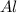
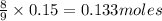 of
of 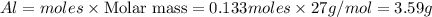
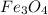
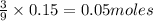 of
of