
Chemistry, 25.01.2021 21:40, 911wgarcia
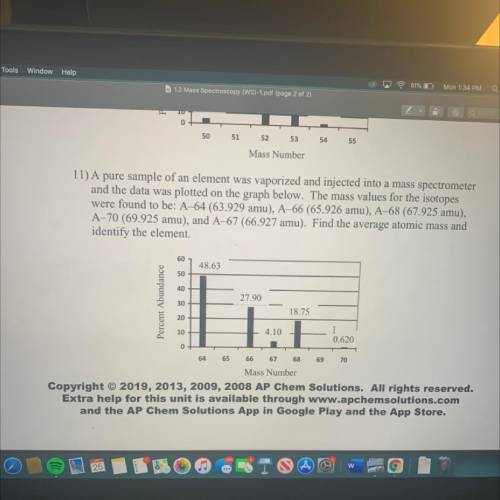
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was plotted on the graph below. The mass values for the isotopes
were found to be: A–64 (63.929 amu), A–66 (65.926 amu), A-68 (67.925 amu),
A-70 (69.925 amu), and A-67 (66.927 amu). Find the average atomic mass and
identify the element.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Do you know the correct answer?
11) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data wa...
Questions in other subjects:

Mathematics, 23.09.2019 16:50


Mathematics, 23.09.2019 17:00

Mathematics, 23.09.2019 17:00




History, 23.09.2019 17:00

Mathematics, 23.09.2019 17:00






