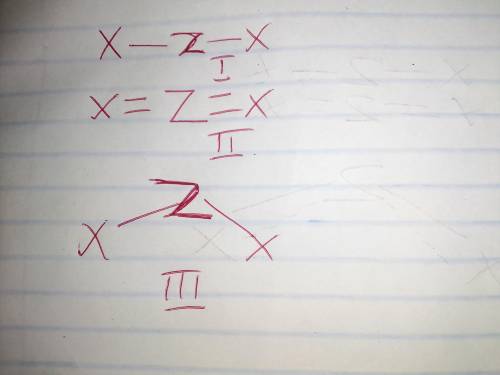Chemistry, 25.01.2021 21:00, juniorlb01
Draw three different Lewis structures that could be possible for ZX2, assuming that Z is the central atom and that X is not hydrogen. Both Z and X obey the octet rule. Give molecular shapes and bond angles for each structure drawn. In each case, use valence shell electron pair repulsion theory to explain why the molecular shape is possible.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1


Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
Do you know the correct answer?
Draw three different Lewis structures that could be possible for ZX2, assuming that Z is the central...
Questions in other subjects:

Mathematics, 16.10.2021 03:50


English, 16.10.2021 03:50

Chemistry, 16.10.2021 03:50

History, 16.10.2021 03:50

Mathematics, 16.10.2021 03:50