
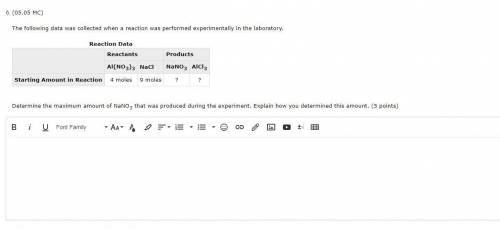
The following data was collected when a reaction was performed experimentally in the laboratory.
Reaction Data
Reactants Products
Al(NO3)3 NaCl NaNO3 AlCl3
Starting Amount in Reaction 4 moles 9 moles ? ?
Determine the maximum amount of NaNO3 that was produced during the experiment. Explain how you determined this amount. (5 points)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, netflixacc0107
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2


Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1
Do you know the correct answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
Re...
Questions in other subjects:


History, 03.07.2019 16:00

Mathematics, 03.07.2019 16:00

Biology, 03.07.2019 16:00


Biology, 03.07.2019 16:00


Mathematics, 03.07.2019 16:00


English, 03.07.2019 16:00






