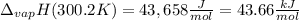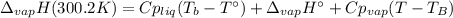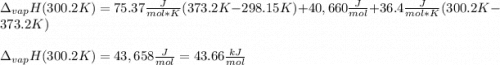Chemistry, 25.01.2021 20:40, PastelHibiscus
The heat of vaporization of water at the normal boiling point, 373.2 K, is 40.66 kJ/mol. The molar heat capacity of liquid water is 75.37 J K-1 mol-1 and that of gaseous water is 36.4 J K-1 mol-1. Assume that these values are independent of temperature. What is the heat of vaporization of water at 300.2 K?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
Do you know the correct answer?
The heat of vaporization of water at the normal boiling point, 373.2 K, is 40.66 kJ/mol. The molar h...
Questions in other subjects:



Social Studies, 26.07.2021 21:20

Mathematics, 26.07.2021 21:20




Mathematics, 26.07.2021 21:20