
Chemistry, 25.01.2021 18:30, jeffcarpenter
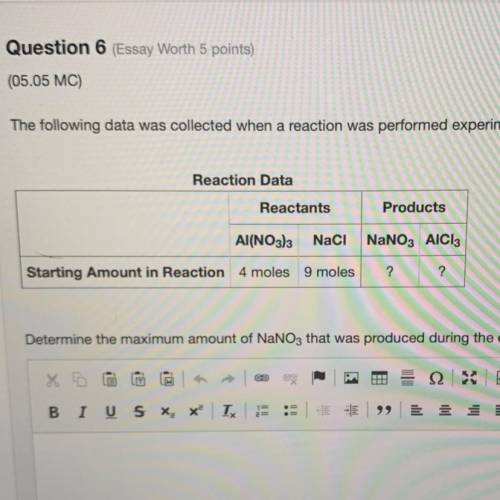
The following data was collected when a reaction was performed experimentally in the laboratory. Determine the maximum amount of NaNO3 that was produced during the experiment. Explain how you determined this amount.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, laurenppylant
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1


Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
Do you know the correct answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
De...
Questions in other subjects:

Business, 23.10.2020 23:30




Physics, 23.10.2020 23:30

Chemistry, 23.10.2020 23:30

Mathematics, 23.10.2020 23:30



Biology, 23.10.2020 23:30






