
Chemistry, 25.01.2021 04:10, 09daishagreen
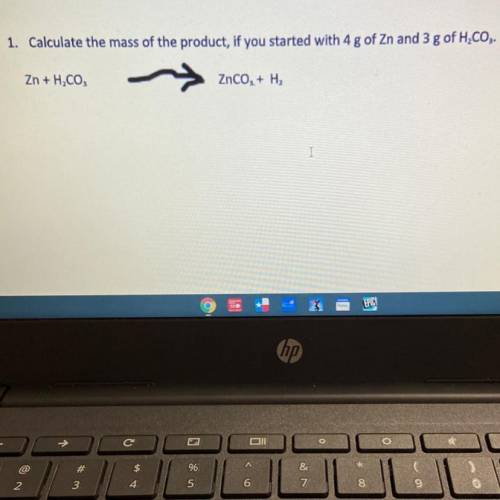
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
ZnCO, + H2

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
Do you know the correct answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
Questions in other subjects:

Mathematics, 26.03.2021 04:30


Mathematics, 26.03.2021 04:30

Mathematics, 26.03.2021 04:30

Chemistry, 26.03.2021 04:30

Chemistry, 26.03.2021 04:30









