Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Do you know the correct answer?
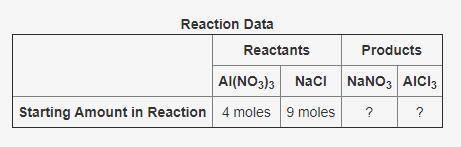
The following data was collected when a reaction was performed experimentally in the laboratory.
De...
Questions in other subjects:

Mathematics, 28.10.2021 01:00

Mathematics, 28.10.2021 01:00


Mathematics, 28.10.2021 01:00





Mathematics, 28.10.2021 01:00

Biology, 28.10.2021 01:00