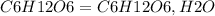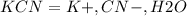Chemistry, 29.10.2019 07:31, cstevenson
The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. each compound is soluble in water.
imagine that a few tenths of a mole of each compound is dissolved in a liter of water. then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. for example, you know water itself will be present, so you can begin each list with the chemical formula for water
note: "major" chemical species are those present in concentrations greater than .
compound formula major species present when dissolved in water
fructose c6h12o6 ¬
potassium cyanide kcn
iron (ii) bromide febr2

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
Do you know the correct answer?
The names and chemical formulae of some chemical compounds are written in the first two columns of t...
Questions in other subjects:

Mathematics, 06.05.2020 08:07


Mathematics, 06.05.2020 08:07