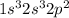Suppose you take a trip to a distant
you find that the periodic table there is derived from a...

Chemistry, 17.10.2019 19:20, jdisalle2808
Suppose you take a trip to a distant
you find that the periodic table there is derived from an arrangement of quantum numbers different from the one on earth. the rules in that universe are:
1. principal quantum number n = 1, 2, . . (as on earth);
2. angular momentum quantum number ℓ = 0, 1, 2,. . , n – 1 (as on earth);
3. magnetic quantum number mℓ = 0, 1, 2, . . , ℓ (only positive integers up to and including ℓ are allowed);
4. spin quantum number ms = –1, 0, 1 (that is, three allowed values of spin).
(a) assuming that the pauli exclusion principle remains valid, what is the maximum number of electrons that can populate a given orbital?
(b) write the electronic configuration of the element with atomic number 8 in the periodic table. (formatting: superscript numbers where appropriate but omit parentheses.)
(c) what is the atomic number of the second noble gas?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deidaralove90
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 13.11.2020 19:50

Chemistry, 13.11.2020 19:50


Computers and Technology, 13.11.2020 19:50


Mathematics, 13.11.2020 19:50

Mathematics, 13.11.2020 19:50


Mathematics, 13.11.2020 19:50