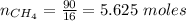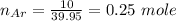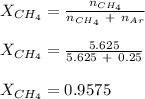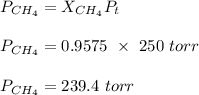Chemistry, 22.01.2021 20:00, ashleybashaam6821
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions of constant temperature and volume. The partial pressure of CH4 in tore is?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2
Do you know the correct answer?
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions o...
Questions in other subjects:




Mathematics, 24.03.2020 21:29

Mathematics, 24.03.2020 21:29