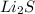Chemistry, 22.01.2021 15:50, christingle2004
The melting points for the compounds Li2S, Rb2S, and K2S are 900, 500, and 840 degrees C, respectively. Give the three compounds in order of increasing lattice energy

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
The melting points for the compounds Li2S, Rb2S, and K2S are 900, 500, and 840 degrees C, respective...
Questions in other subjects:



Mathematics, 15.01.2021 21:40

Mathematics, 15.01.2021 21:40

Chemistry, 15.01.2021 21:40

Mathematics, 15.01.2021 21:40


Mathematics, 15.01.2021 21:40


Physics, 15.01.2021 21:40


 <
<  <
<