Chemistry, 17.10.2019 12:30, alexisthegirl
Careful spectral analysis shows that the familiar yellow light of sodium lamps (such as street lamps) is made up of photons of two wavelengths, 589.0 nm and 589.6 nm. what is the difference in energy (in joules/mol) between one mole of photons with these wavelengths?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
Do you know the correct answer?
Careful spectral analysis shows that the familiar yellow light of sodium lamps (such as street lamps...
Questions in other subjects:

Biology, 31.07.2020 22:01


Chemistry, 31.07.2020 22:01


Social Studies, 31.07.2020 22:01

Mathematics, 31.07.2020 22:01

Chemistry, 31.07.2020 22:01


Health, 31.07.2020 22:01


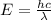
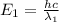
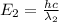
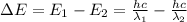
![\Delta E=hc\times [\frac{1}{\lambda_1}-\frac{1}{\lambda_2}]](/tpl/images/0328/2709/df32b.png)
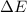 = difference in energy of photon = ?
= difference in energy of photon = ?
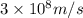
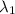 = wavelength of photon 1 = 589.0 nm =
= wavelength of photon 1 = 589.0 nm = 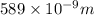
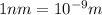
 = wavelength of photon 2 = 589.6 nm =
= wavelength of photon 2 = 589.6 nm = 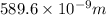
![\Delta E=(6.626\times 10^{-34}Js)\times (3\times 10^8m/s)\times [\frac{1}{589\times 10^{-9}m}-\frac{1}{589.6\times 10^{-9}m}]](/tpl/images/0328/2709/ce225.png)