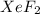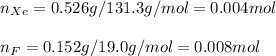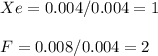Chemistry, 21.01.2021 22:00, shongmadi77
A compound containing xenon and fluorine was prepared by shining sunlight on a mixture of Xe (0.526 g) and excess F2 gas. If you isolate 0.678 g of the new compound, what is its empirical formula

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
Do you know the correct answer?
A compound containing xenon and fluorine was prepared by shining sunlight on a mixture of Xe (0.526...
Questions in other subjects:

Mathematics, 19.02.2021 02:20

Mathematics, 19.02.2021 02:20

Social Studies, 19.02.2021 02:20

Mathematics, 19.02.2021 02:20


Mathematics, 19.02.2021 02:20

Mathematics, 19.02.2021 02:20