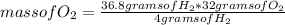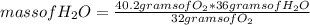Chemistry, 21.01.2021 22:00, jasmine3051
How many grams of H2O will be formed when 36.8 g H2 is mixed with 40.2 g O2 and allowed to completely react to form water

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, lemonsalt9378
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
How many grams of H2O will be formed when 36.8 g H2 is mixed with 40.2 g O2 and allowed to completel...
Questions in other subjects:


Mathematics, 20.04.2020 13:50


History, 20.04.2020 13:50

Chemistry, 20.04.2020 13:50


Mathematics, 20.04.2020 13:50


History, 20.04.2020 13:50

Health, 20.04.2020 13:50