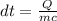If 63.4 J of heat are added to a sample
of aluminum with a mass of 16.33 g, what is
its tempe...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Medicine, 30.08.2019 19:10




Mathematics, 30.08.2019 19:10



Mathematics, 30.08.2019 19:10