
Chemistry, 21.01.2021 15:40, cxttiemsp021
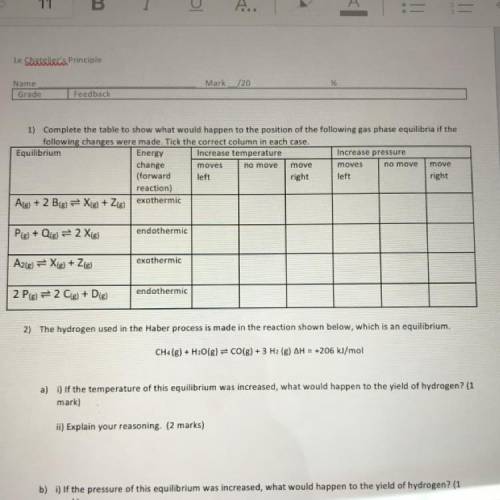
1) Complete the table to show what would happen to the position of the following gas phase equilibria if the
following changes were made. Tick the correct column in each case.
Equilibrium
Energy Increase temperature
Increase pressure
change
moves
moves
move
(forward left
right left
right
reaction)
Ale) + 2 B(g) = X(g) + Zig) exothermic
endothermic
Ple) + Qig) = 2 Xig)
exothermic
A2(g) = X(g) + 2(g)
endothermic
2 Pig) = 2 C(s) + D(g)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
Do you know the correct answer?
1) Complete the table to show what would happen to the position of the following gas phase equilibri...
Questions in other subjects:



Social Studies, 06.12.2019 06:31













