

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Do you know the correct answer?
13.How many grams of phosphorus (P4) are needed to completely consume 79.2 L of chlorine gas accordi...
Questions in other subjects:

Biology, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Engineering, 04.10.2021 14:00


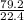 = 3.54mole
= 3.54mole 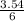 = 0.59mole of P₄
= 0.59mole of P₄ 



